Lecture 14: Chemical Potential
Note
Entropy is the price of structure. – Ilya Prigogine
Warning
This lecture corresponds to Chapter 22 of the textbook.
Summary
Attention
In this chapter, we consider another natural variable: the number of particles in a given system. Just like we did at the beginning of our journey in this course, we will start with the internal energy and introduce a new concept that will enable us to consider how changes in particle number affect internal energy. Simply, we introduce the chemical potential which is the energy required to add a particle to the system:
We see that the chemical potential is formally defined as:
This can be used to find the useful relationship:
Just like we did before in Lecture 8: Thermodynamic potentials, we can use various constraints and the formal description shows that:
In turn, based on results we obtained in Lecture 13: Statistical mechanics of an ideal gas, we see that for an ideal gas:
Further manipulations on functions of state lead us to this other definition:
The attentive student will notice that we are now dealing with slightly more complicated functions of state. This comes from the fact that we now need three natural variables to describe a given state. For instance, entropy, volume, and number of particles are the natural variables used to describe the total internal energy. One can also introduce a new function of state, known as the Grand Potential. It is built similarly to the way one built the Helmholtz function but this time by also making sure that the natural variable is the chemical potential (rather than the number of particles):
The most important concept students will remember from this chapter is that the way the particles are transferred between two subsystems is governed by the second law of thermodynamics: the system will evolve (that is: exchange particles) until the chemical potential is uniform. It is useful to make a formal comparison with the case of two systems that are initially at different temperatures: when put in contact, the two systems will exchange heat until the temperature is uniform.
We will also remember that the chemical potential can be thought of as the Gibbs function per particle.
This formalism now allows us to study chemical reactions. In particular, we find that for an ideal gas:
where we defined standard pressure and temperature conditions using the symbol””
For a chemical reaction, the equilibrium constant is obtained in terms of the change in Gibbs function:
And, using the Gibbs-Helmholtz relation, we have:
This last equation formalizes Le Chatelier’s principle: a system
at equilibrium will respond to a disturbance in such a way as to
minimize that disturbance. Indeed, for an endothermic process, an
increase in temperature will lead to an increase in the amount of
products (larger ).
Finally, we introduce the concept of entropic forces, of which a couple of examples are illustrated in the blue boxes below.
Entropic versus energetic spring
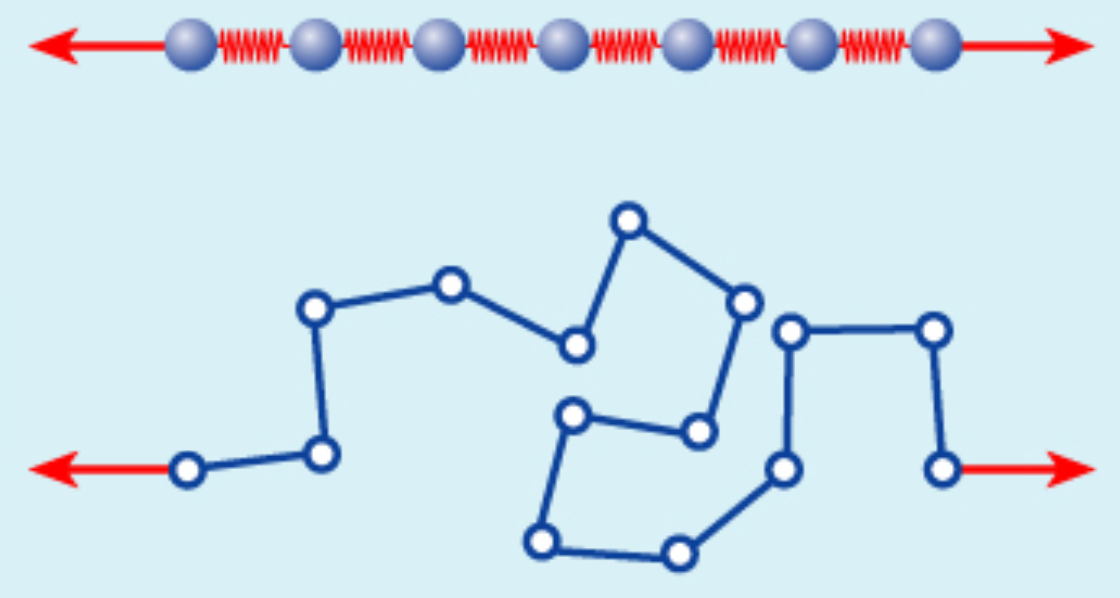
The example shown in the image is an illustration of the difference between a ‘classical’ and an ‘entropic’ force. In the first case, stretching the one-dimensional system is governed by Hooke’s law for each spring. In the second case, the difficulty to stretch the disordered spring comes from the fact that it is in a disordered configuration (with many microstates, and thus high entropy); stretching it would decrease the entropy.
Osmosis
Osmosis is an illustration of the concept of entropic force. It is best understood using the figure below:
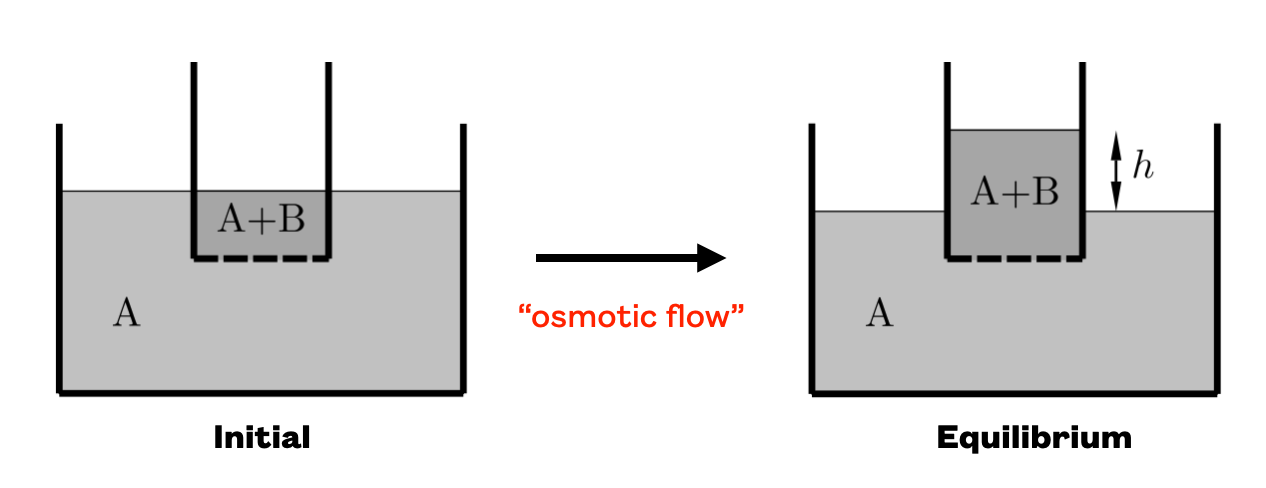
First, note that the small container has, at its bottom, a
semi-permeable membrane (shown by dashed lines) that only allows
the transport of ‘A’ components while ‘B’ are being blocked. The reason why
the system evolves as it does, to the right, is due to the fact
that the situation with the diluted “B” component corresponds to a
much larger number of microstates than the initial situation. So,
why does it stop at height ? Well, the column A+B will need
to remain in equilibrium with the external pressure and the
equilibrium will be reached when the pressure due to gravity is
identical to the so called Osmotic pressure:
The number of applications of osmosis is large and of critical importance, especially in biology. Clearly there would be no life forms as we know them without the existence of entropy. Isn’t thermodynamics a wonderful subject?
Key Definitions
Note
- Chemical potential:
the chemical potential is the amount of energy added to a system when an additional particle of a given type is added to it; it is usually noted
.
- Equilibrium constant:
For a chemical reaction, one can write in all generality that the equilibrium constant is
where the
’s are the partial pressure of product/reactant
. The
’s are the coefficients of the reaction: they are conventionally chosen as negative (reactants) and positive (products).
- Micro canonical ensemble:
this is an ensemble of systems with the same energy.
- Canonical ensemble:
ensemble of systems exchanging energy with a large heat reservoir; as a result these systems are held at constant temperature. This is the type of ensembles for which one can use the Boltzmann distribution.
- Grand canonical ensemble:
ensemble of systems that can exchange both energy and particles with a large reservoir (this fixes temperature and chemical potential). For this type of systems, one uses Gibbs’ probability distribution.
- Endothermic:
an endothermic reaction is a reaction that takes in heat. Using our convention, this means a positive heat exchange. This has to be contrasted with an Exothermic reaction, which releases heat (which takes on a negative sign).
- Gibbs’ probability distribution:
This distribution applies to grand canonical ensembles:
where
is the Grand Partition Function.
- van ‘t Hoff equation:
This equation provides a relationship for the change of equilibrium constant with temperature:
This equation clearly shows that the slop is proportional to the change in enthalpy under standard conditions.
- Le Chatelier principle:
This principle establishes that a system at equilibrium, when subjected to a disturbance, responds in such a way as to minimize that disturbance.
A full list of terms, including the ones provided here, can be found in the Index.
Learning Material
Copy of Slides
The slides for Lecture 14 are available in pdf format here: pdf
Screencast
Test your knowledge
- Among the definitions below, which one describes the concept of chemical potential the most closely?
The chemical potential represents the probability of a chemical reaction to take place.
The chemical potential quantifies the change in internal energy when one particle is added to a system.
The chemical potential is the heat transferred from (or to) a system when a chemical reaction takes place.
The chemical potential is the largest amount of work a system can do as the result of a complete chemical reaction.
- When considering a process (such as a chemical reaction) when the number of particle
changes, it is usual to use
as a natural variable.
This is true even if we understand that calculating derivatives with respect to an integer value such as
can give some headache to mathematicians.
This is true but we usually do not do this since the change in energy or entropy when the number of particles changes can be neglected.
This is not true since the first law of thermodynamics does not include cases where the number of particles can change.
I think I know what is going on but I’m not sure what the right answer is. I will therefore choose this option even though this would yield zero points for this question.
- When considering a process (such as a chemical reaction) when the number of particle
- The second law of thermodynamics is clear: the entropy of a closed system always increases. Imagine two subsystems kept at the same temperature
. The chemical potential on the left is
and the chemical potential on the right is
mu_1`. You now allow particles to move between the two subsystems. What is the most likely scenario? (to answer this question, remember that
where
is the number of particles moving from the left to the right.)
Particles will flow from the left to the right.
Particles will flow from the right to the left.
Nothing happens since the temperature is uniform.
- The second law of thermodynamics is clear: the entropy of a closed system always increases. Imagine two subsystems kept at the same temperature
- What is a grand canonical ensemble?
That’s a great question. Thanks for asking it.
It is an ensemble with fixed temperature and chemical potential.
It is an ensemble with fixed temperature and number of particles.
It is an ensemble with fixed energy and temperature.
- What is the relation between the Gibbs function and the chemical potential?
There is no connection between them.
The Gibbs function is the first derivative of the chemical potential with respect to temperature.
The chemical potential is the Gibbs function per particle.
- The number of photons in vacuum is not a conserved quantity. Thus, the second law of thermodynamics translates into the fact that…
Their chemical potential is arbitrary.
The number of photons will adjust until the chemical potential reaches zero at equilibrium.
The chemical potential is conserved.
- What do we mean when we refer to standard values for pressure and temperature?
We refer to the instructor’s favorite soccer team in Belgium.
We refer to pressure and temperature conditions typically found in a chemistry laboratory (i.e.,
atm and
).
We refer to pressure and temperature values when a chemical reaction takes place.
- In the context of chemical reactions, what do we mean when we use the term molar?
We refer to quantities that are expressed in per mole units
We refer to quantities that are expressed in per molecule units
We refer to the fact students usually want to bite their thermodynamics homework all the way until the deadline for submission.
- Consider the following equation:
where
is the equilibrium constant and the other variables are used in a way similar as we have done so far in this course. Based on that equation, what is the most accurate statement?
For an endothermic reaction, the reaction leans more to the right as
increases.
For an exothermic reaction, the reaction leans more to the right as
increases.
There is not enough information to draw definitive conclusions on this question.
- Consider the following equation:
- Jacobus van ‘t Hoff…
wrote an equation that provides a link between the reaction rate and its enthalpy change.
is a distant cousin of Stephen van Rensselaer and his equation states that more than 2 particles occupying a given container at (hopefully!) different times are more likely to cause a pandemic than 30 particles seated in a medium size container at the same time.
is a distant cousin of van der Waals but he worked on the hard-sphere potential rather than weak bonding.
None of the other answers is correct.
- The concept of entropic force originates from
the gradient of the entropic potential.
the second law of thermodynamics and the maximization of entropy.
the need to give fancy names to elementary problems we learned in kindergarten.
None of the other answers is correct.
- The existence of osmosis and the fact rubber cools when elongated are manifestations of…
The fact physics never ceases to amaze me.
Entropy can have macroscopic effects, including the emergence of forces that sometimes behave counter-intuitively.
The third law of thermodynamics, as the absolute zero of temperature cannot be met.
Two separate effects, both related to thermodynamics, but with nothing else in common.
Hint
Find the answer keys on this page: Answers to selected test your knowledge questions. Don’t cheat! Try solving the problems on your own first!