Lecture 5: Second Law of Thermodynamics
Note
Nature prefers the more probable states to the less probable because in nature processes take place in the direction of greater probability. Heat goes from a body at higher temperature to a body at lower temperature because the state of equal temperature distribution is more probable than a state of unequal temperature distribution. — Max Planck
Warning
This lecture corresponds to Chapter 13 of the textbook.
Summary
Attention
- Second law of thermodynamics (two equivalent statements):
Clausius: No process is possible whose sole result is the transfer of heat from a colder to a hotter body.
Kelvin: No process is possible whose sole result is the complete conversion of heat into work.
Later, we will describe a third, equivalent, statement of the second law of thermodynamics that states that in a thermally isolated system, all real process leads to an increase in entropy (see Lecture 6: Entropy)
The main topic of discussion in this chapter will be the Carnot cycle. The Carnot cycle is a reversible cycle that consists of an isotherm, an adiabat, a second isotherm, and a second adiabatic process to complete the cycle. The process takes place between a hot and cold temperature reservoirs. Work is released as the difference between the heat transferred from the hot reservoir and the heat transferred to the cold reservoir.
A lot of information can be obtained from a detailed study of the Carnot cycle. One key finding is Clausius’ inequality, which states that:
For a reversible cycle, we have:
This is a key finding as the last equation indicates the existence of an exact differential! This is the topic of Lecture 6: Entropy.
Learning Material
Copy of Slides
The slides for Lecture 5 are available in pdf format here: pdf
Screencast
Key Definitions
Note
- Heat engines:
machines that produce work from a temperature difference between two reservoirs.
- Reservoir:
a large body that keeps its temperature regardless of the amount of heat taken away from it (i.e., infinite heat capacity).
- Engine:
system operating a cyclic process that converts heat into work. It has to be cyclic so that it can be continuously operated, producing a steady power.
- Carnot cycle:
thermodynamic cycle operating between two different heat reservoirs operating with 4 reversible processes: isotherm, adiabat, isotherm, and adiabat.
- Otto cycle:
thermodynamic cycle operating between two different heat reservoirs operating with 4 reversible processes: adiabat, isochore, adiabat, isochore.
- Engine efficiency:
ratio between heat pumped from a hot reservoir and the total work produced (symbol:
).
- Isochore:
an isochore process is one that occurs at constant volume.
- Isobar:
an isobar process is one that occurs at constant pressure.
- Isentropic:
an isentropic process is one that occurs at constant entropy (that is: isothermal and reversible).
A full list of terms, including the ones provided here, can be found in the Index.
Test your knowledge
- The second law of thermodynamics establishes that
No process is possible whose sole result is the transfer of heat from a colder to a hotter body.
No process is possible whose sole result is the complete conversion of work into heat.
More than one answer provided is correct.
None of the other options is correct.
- The Carnot cycle must operate between two different temperatures.
True.
False.
It depends.
- What is true about the efficiency of the Carnot cycle?
It is the smallest efficiency possible of all heat engines operating between two given temperatures.
Cannot be 100%. This fact is an expression of the second law of thermodynamics.
Cannot be 100%. This fact is an expression of the first law of thermodynamics.
- What is the most accurate statement regarding Clausius inequality?
Clausius inequality states that the absolute zero of temperature cannot be reached.
Is always a strict inequality, even for a reversible cycle.
Is always a strict inequality, unless we have a reversible cycle.
- A heat pump…
Is a heat engine operating in the opposite direction than a typical heat engine. Its efficiency is at most 100%.
Is a heat engine operating in the opposite direction than a typical heat engine. Its efficiency is always larger than 100%.
Consider the cycle drawn in the figure…
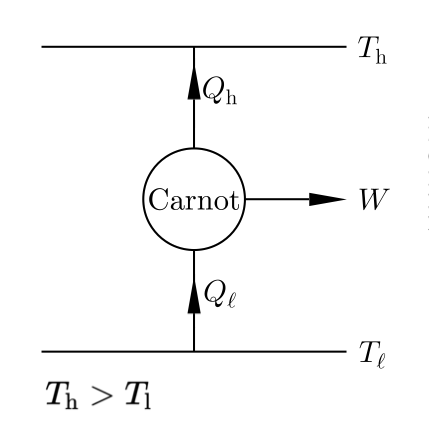
There is nothing wrong with this picture, this is the Carnot Engine.
There is nothing wrong with this picture, this is the Carnot Engine running in reverse (i.e., a refrigerator).
This engine violates the first law of thermodynamics (conservation of energy).
This engine violates the second law of thermodynamics (Clausius’ version only).
This engine violates the second law of thermodynamics (Kelvin’s version only).
None of the other answers is correct.
- During the Carnot cycle, work is done in both the adiabatic and isothermal processes. In this question, we will focus on the work involved in the adiabatic process. Select the most accurate statement below:
No work is done during the adiabatic process.
Work is done by the system during the expansion and done on the system during the compression. In absolute value, the two works are identical.
Work is done by the system during the compression and done on the system during during the expansion. In absolute value, the two works are identical.
Work is done by the system during the expansion and done on the system during the compression. In absolute value, the work involved during expansion is larger than the work involved during compression.
Work is done by the system during the expansion and done on the system during the compression. In absolute value, the work involved during expansion is smaller than the work involved during compression.
None of the other answers is correct.
Hint
Find the answer keys on this page: Answers to selected test your knowledge questions. Don’t cheat! Try solving the problems on your own first!